Our takeaways:
- FDA revised the pregnancy and breastfeeding labeling format on prescription and biological drugs to replace the old A, B, C, D and X categories but labeling for over-the-counter (OTC) medicines are not affected by this change.
- While fewer than 10% of medications have enough information to determine fetal risks, there are a list of reliable tools to help pregnant/breastfeeding women and their health providers make informed decisions. i.e: MotherToBaby Fact Sheets, text4Baby, Treating for Two, etc.
- The general rule of thumb for medications during pregnancy & breastfeeding:
- There are other factors (than the medications themselves) that would impact the risk level to you and your baby.
- It’s usually best to choose one that has only one active ingredient (instead of many active ingredients).
- “Natural” does not mean safe.
- We summarized the most commonly used OTC medications and their risks (if any) for pregnancy & breastfeeding at the end of this article.
Always consult your healthcare provider for medical advice about drug use while pregnant or breastfeeding. The information summarized here is for reference only, and should not take the place of medical care and advice from your healthcare provider.
If you have been planning for pregnancy or just became (expectant/) parents, you probably have collected a list of risky foods for your baby(-ies). For example, you’d want to stay away from high-mercury fish and deli meats (See our pregnancy lifestyle vision board for more). But what about the Tylenol that you usually take to combat flu, ibuprofen for muscle aches, Tums for indigestion, or the prescribed drugs you got prior to pregnancy? Are they risky for the fetus and/or the mom? If so, how risky?
Almost every pregnant woman will face a decision about taking medicines before and during pregnancy or postpartum (if they choose to breastfeed). In the United States, 9 out of 10 women take at least one medicine during pregnancy. Unfortunately, fewer than 10% of medications have enough information to determine fetal risks (i.e: pregnancy loss, prematurity, birth defects, infant death, developmental disabilities, etc). As a result, the medication decisions haven’t been easy for pregnant and breastfeeding women and their health providers.
So as parents/parents-to-be, how do we make informed decisions given the limited information available? How confident should we be based on this available information? In this article, we will dig into reliable (and free!) tools that can help answer these questions.
Reliable tools for medication safety during pregnancy & breastfeeding
1. The “A, B, C, D and X risk categories” by FDA (U.S. Food and Drug Administration)
If you have read a pregnancy book or two, you might have encountered this “A, B, C, D and X risk categories” by FDA. For example, in her famous Expecting Better, author Emily Oster spent the entire Chapter 13 on this. While somewhat informative, this letter categorization in use since 1979 is reportedly leaving users and their health providers ill-informed and resulting in false assumptions about the actual meaning of the letters.
In 2015, FDA revised the pregnancy and breastfeeding labeling format on prescription and biological drugs through the “Pregnancy and Lactation Labeling Rule” (PLLR or final rule). The new labels replaced the old A, B, C, D and X categories with more helpful narratives on a medicine’s risks, and have more information on whether the medicine gets into breast milk and how it can possibly affect the baby (Source). Note: labeling for over-the-counter (OTC) medicines are not affected by this change.
Specifically, the changes can be found in each medication’s label section 8 “Use in Specific Population”. Below is a comparison of the current prescription drug labeling with the new PLLR labeling requirements.
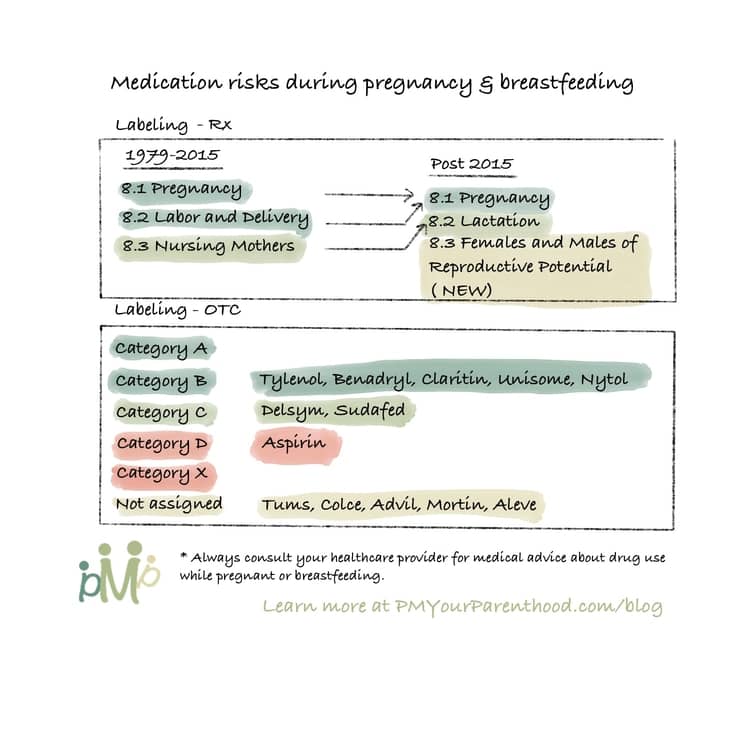
The Pregnancy subsection will provide information about dosing and potential risks to the developing fetus and registry information that collects and maintains data on how pregnant women are affected when they use the drug or biological product. Information in drug labeling about the existence of any pregnancy registries has been previously recommended but not required until now. Contact information for the registries will also be included, and pregnant women are encouraged to enroll to help provide data on the effects of drug use or biologics in pregnancy.
If the information for the subsections of Pregnancy Exposure Registry, Clinical Considerations, and Data is not available, these subsections will be excluded. The Risk Summary subheadings are always required, even if no data is available.
The Lactation subsection will replace the “Nursing Mothers” subsection of the old label. Information will include drugs that should not be used during breastfeeding, known human or animal data regarding active metabolites in milk, as well as clinical effects on the infant. Other information may include pharmacokinetic data like metabolism or excretion, a risk and benefits section, as well as the timing of breastfeeding to minimize infant exposure.
In the subsection entitled Females and Males of Reproductive Potential, relevant information on pregnancy testing or birth control before, during or after drug therapy, and a medication’s effect on fertility or pregnancy loss will be provided when available.
This new labeling enables pregnant women and their providers to have transparent discussions and make informed decisions when prescription or biological medications are necessary.
2. MotherToBaby Fact Sheets recommended by CDC (U.S. Centers for Disease Control and Prevention)
Having understood the risk labeling, you might still find it daunting in finding reliable information on specific medications (prescription or OTC). In addition to FDA’s Medication Guides, CDC also recommended checking MotherToBaby Fact Sheets for the risks and safety of taking specific medicines during pregnancy and breastfeeding.
What I like about the Fact Sheets is that they are well categorized, sorted and searchable. All medications are listed by generic name. To locate the generic name of your medication, look for the ‘Active Ingredient’ listed on your medication bottle or packaging. Also, you can usually find generic names in parentheses after the medication’s brand name. In addition to medications, the Fact Sheets also cover a wide range of other “categories of exposure” such as Cosmetic Treatment, Environment & Natural Disasters, Infections & Vaccines, etc.
3. text4baby recommended by CDC
Text4baby sends free text messages to pregnant women & moms with health & safety info. What I like about it is that the messages are timed to either mom’s due date or baby’s birthday for a personalized auto-reminder tailored to your and your baby’s progress. Especially for a first-time mom who’s probably overwhelmed by all kinds of new knowledge, this feature is definitely a huge time saver that helps you focus on the info that only matters to your current stage.
Text4baby also extended its service from text messages to apps (still free), with a 4.6 out of 5 stars in the Apple App store.
4. “Treating for Two” program by CDC
Treating for Two is a program that aims to improve the health of women and babies by identifying the safest treatment options for common conditions before, during, and after pregnancy. In my opinion, this site serves best when you have known health conditions and are wondering how pregnancy or breastfeeding would impact your treatment. It provides a selection of detailed research and studies on specific conditions to help you make informed decisions with your health providers.
5. Drugs and Lactation Database (LactMed)
The LactMed® database contains information on drugs and other chemicals to which breastfeeding mothers may be exposed. It includes information on the levels of such substances in breast milk and infant blood and the possible adverse effects on the nursing infant. Suggested therapeutic alternatives to those drugs are provided, where appropriate. All data are derived from the scientific literature and fully referenced. A peer review panel reviews the data to assure scientific validity and currency.
6. e-lactancia recommended by AELAMA of Spain
AELAMA is the Spanish Association for the Promotion and Support of Breastfeeding. The e-lactancia tool is a gigantic and continuously updating database that enables nursing parents to check the compatibility of breastfeeding with 32,837 terms (as of 8/28/2022), including medications, plants, fruit, seeds, etc that we might encounter in our daily lives.
Compared to LactMed®, e-lactancia supports Spanish in addition to English, which is a huge plus to the Spanish-speaking communities. Also, e-lactancia uses color coding to distinguish risk levels and make content easier to digest and decision-making more straightforward: (See detailed definition on their website)
- Green – Very low risk.
- Light Orange – Low risk.
- Orange – High risk.
- Red – Very high risk.
The general rule of thumb for medications during pregnancy & breastfeeding
Complementing the reliable tools above and directions from your healthcare providers, here is a helpful rule of thumb to keep in mind when considering medications during pregnancy and breastfeeding:
1. There are other factors (than the medications themselves) that would impact the risk level to you and your baby.
These factors include:
- How much medicine you take (aka the dose),
- When during the pregnancy (1st, 2nd, or 3rd trimesters) you take the medicine,
- Other health conditions you have, and
- Other medicines you take.
2. It’s usually best to choose one that has only one active ingredient (instead of many active ingredients).
If you select an over-the-counter medication as recommended by your doctor, it’s usually best to choose one that has only one active ingredient (instead of many active ingredients). Selecting a single-ingredient product with only the active ingredient you need helps to limit the exposure to medicines in your baby.
For example, if you have a cough, instead of using a liquid cough suppressant that also contains acetaminophen (Tylenol), just use a product that contains only the cough medicine. Ask your pharmacist for help with this decision, if needed.
3. “Natural” does not mean safe.
Over-the-counter “natural” dietary supplements may contain unknown substances and few studies describing their use in pregnancy are available. “Natural” does not mean safe. In general, it is best to avoid OTC herbal and certain dietary supplements in pregnancy. These products are not regulated by the FDA and may have variable quality, be contaminated, or contain undeclared prescription medicines.
4. Here’s an easy reference for the most commonly used OTC medications and their risks (if any) for pregnancy & breastfeeding. (Ordered by Medication name alphabetically)
| Medication | Brand Name(s) | Usage | Pregnancy Indications | Breastfeeding Indications | |
| Acetaminophen | Tylenol | Pain, headache and fever treatment | Category B. Use only as needed and at the lowest effective dose (source) | Amounts in milk are much less than doses usually given to infants. Adverse effects in breastfed infants appear to be rare. (source) | |
| Aspirin | Bayer Aspirin | Reduces inflammation, fever, and pain; prevent blood clots | Category D. Regular strength and high strength aspirin are NOT preferred pain relievers during pregnancy. (source) | An alternate drug is preferred over continuous high-dose, aspirin therapy. If low-dose aspirin is used by a nursing mother, monitor the infant for bruising and bleeding. (source) | |
| Calcium Carbonate | Tums, Rolaids, Mylanta | An antacid to help symptoms of heartburn, acid indigestion, or upset stomach | Not been formally assigned to a pregnancy category by the FDA. Taking at recommended levels is not expected to increase the chance for birth defects. (source) | When calcium carbonate is taken at recommended doses, it is unlikely to be harmful to a nursing baby. (source) | |
| Dextromethorphan | Delsym | Cough suppresant | Category C. Dextromethorphan should only be given if the benefit to the mother justifies the potential risk to the fetus as determined by your doctor. (source) | The amounts of dextromethorphan and its active metabolite in breastmilk are very low and are not expected to affect the nursing infant. (source) | |
| Diphenhydramine | Benadryl, Unisom, Nytol | Allergy symptoms, and may also be used to treat nausea, motion sickness, insomnia, itchy skin, and tremor of Parkinson’s disease | Category B. Should be used during pregnancy only if clearly needed. (source) | Small, occasional doses would not be expected to cause any adverse effects in breastfed infants. Larger doses or more prolonged use may cause effects in the infant or decrease the milk supply. (source) | |
| Docusate | Colace | Stool softener/constipation treatment | Not been formally assigned to a pregnancy category by the FDA. When used in recommended doses docusate sodium is not expected to increase the chance of birth defects (source) | Docusate is minimally absorbed from the gastrointestinal tract and therefore the drug is unlikely to be found in the maternal serum or breastmilk. Laxatives that are completely unabsorbed may be preferred. (source) | |
| Loratadine | Claritin | Lessen the effects of allergic reactions and colds. Treat symptoms such as sneezing, runny nose, watery eyes, an itchy throat, and an itchy rash or hives. | Category B. It is unlikely that taking loratadine would increase the chance for birth defects. (source) | Not expected to cause any adverse effects in breastfed infants. When at its lowest dose, it’s a preferred antihistamine during breastfeeding. (source) | |
| NSAIDs incl. Ibuprofen, naproxen | Advil, Mortin, Aleve | Antiinflammatory drugs for pain and fever | Not been formally assigned to a pregnancy category by the FDA. Avoid the use of NSAIDs at 20 weeks of pregnancy and later due to rare but serious kidney problems in an unborn baby. (source) | Ibuprofen: Extremely low levels in breastmilk; a preferred analgesic or anti-inflammatory agent in nursing mothers (source) Naproxen: other agents may be preferred while nursing a newborn or preterm infant due to its long half-life and reported serious adverse reaction in a breastfed neonate. | |
| Pseudoephedrine | Sudafed | Nasal congestion by cold or allergy; pain medications | Category C. NOT the preferred treatment for congestion. Some studies have found a small increased chance for specific birth defects. i.e: gastroschisis, small intestinal atresia, etc. | May decrease milk supply. Mothers with newborns whose lactation is not yet well established or mothers who are having difficulties producing sufficient milk should not receive pseudoephedrine. (source) |
Like this article? Share it with your loved ones! To get bite-size parenthood wisdom like this, or save infographics for future references, follow us on Instagram (@PMYourParenthood)!
Also you can support me and my work by contributing a cup of coffee here:


